Mastering Molecular And Electron Geometry: A Guide To Understanding Shape And Structure
In the realm of chemistry, molecular and electron geometry are crucial concepts that help us decipher the three-dimensional shape of molecules. These geometries are pivotal in determining the properties and behavior of molecules, influencing everything from their reactivity to their interactions with other molecules. Understanding these geometries allows scientists to predict how molecules will behave in different environments, making them essential for fields such as pharmacology, materials science, and environmental chemistry.
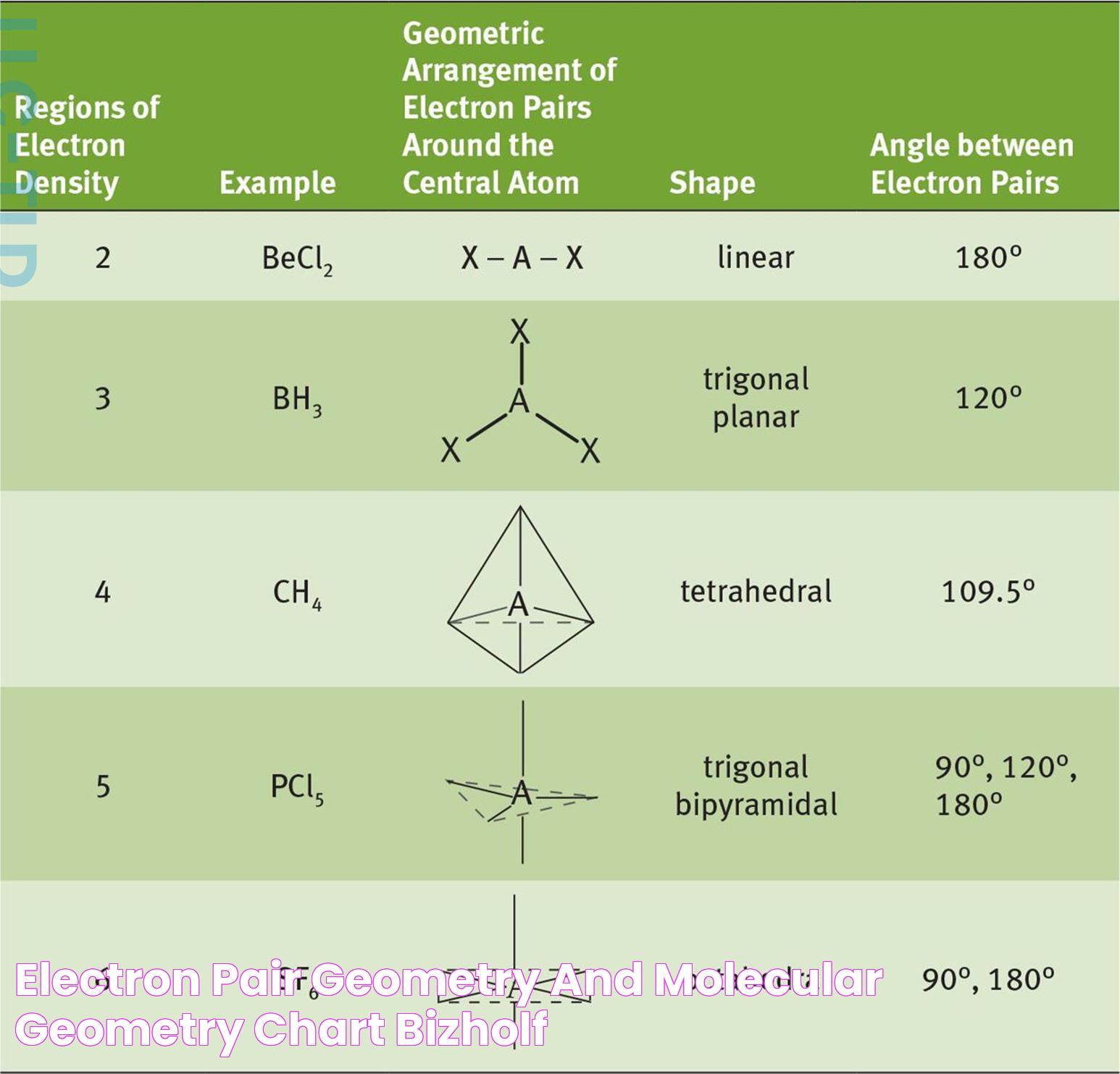
The study of molecular and electron geometry involves examining both the arrangement of atoms within a molecule and the spatial distribution of electron pairs. Molecular geometry describes the shape formed by the atoms in a molecule, while electron geometry considers the arrangement of all electron pairs, both bonding and non-bonding. These geometries are often explained using the Valence Shell Electron Pair Repulsion (VSEPR) theory, which provides a model for predicting molecular shapes based on the repulsion between electron pairs.
For students and professionals alike, mastering molecular and electron geometry is a fundamental step toward a deeper understanding of molecular structure and function. By exploring these geometries, one can gain insights into how molecules interact, how they form bonds, and how their shapes influence their chemical and physical properties. This guide aims to provide a comprehensive overview of molecular and electron geometry, offering a valuable resource for anyone looking to expand their knowledge in this fascinating area of chemistry.
Read also:El Tiempo En Houston Guiacutea Completa Del Clima Y Las Estaciones
Table of Contents
- What is Molecular Geometry?
- What is Electron Geometry?
- The VSEPR Theory: A Foundation for Geometry
- How Do Bond Angles Influence Geometry?
- Tetrahedral Geometry: A Common Shape
- Linear Geometry: Simplicity in Structure
- Trigonal Planar Geometry: A Flat Arrangement
- Bent Geometry: The Influence of Lone Pairs
- Octahedral Geometry: A Six-Pointed Configuration
- Trigonal Bipyramidal Geometry: An Expanded View
- Molecular Geometry in Complex Molecules
- How Does Geometry Affect Molecular Polarity?
- Applications of Molecular Geometry in Real-World Scenarios
- Challenges in Determining Molecular Geometry
- FAQs
- Conclusion
What is Molecular Geometry?
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. This geometric arrangement is crucial as it determines the molecule's properties, such as polarity, reactivity, phase of matter, color, magnetism, and biological activity. Molecular geometry can be predicted using various models and theories, with the VSEPR theory being one of the most widely utilized methods.
Molecular geometry is characterized by specific shapes, such as linear, bent, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. Each shape has distinct bond angles that result from the repulsion between electron pairs surrounding a central atom. This geometry is particularly important in understanding how molecules interact with each other and how they participate in chemical reactions.
For instance, the geometry of water (H₂O) is bent due to the presence of two lone pairs on the oxygen atom, which results in a bond angle of about 104.5°. This shape contributes to water's unique properties, such as its high surface tension and boiling point. Understanding molecular geometry is essential for predicting and explaining the behavior of molecules in various chemical contexts.
What is Electron Geometry?
Electron geometry, on the other hand, considers the spatial arrangement of all electron pairs (bonding and non-bonding) around a central atom. It provides a broader perspective of a molecule's structure, focusing on the electron clouds that contribute to the overall shape. Electron geometry is crucial in determining the distribution of electron density, which influences molecular properties.
Unlike molecular geometry, which focuses on the arrangement of atoms, electron geometry includes lone pairs and multiple bonds. For example, the electron geometry of ammonia (NH₃) is tetrahedral, as it accounts for the three hydrogen atoms and one lone pair around the nitrogen atom. However, the molecular geometry is trigonal pyramidal, emphasizing the spatial arrangement of the hydrogen atoms only.
Understanding electron geometry is fundamental for predicting molecular behavior, as electron repulsions play a significant role in shaping molecules. It provides insights into how molecules will interact with other substances, offering a more comprehensive understanding of molecular properties and functions.
Read also:How Many People Can You Gameshare With On Ps5 A Comprehensive Guide
The VSEPR Theory: A Foundation for Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone in the study of molecular and electron geometry. This theory proposes that the shape of a molecule is determined by the repulsion between electron pairs in the valence shell of the central atom. By minimizing repulsion, the molecule adopts a specific geometry that allows electron pairs to be as far apart as possible.
According to VSEPR theory, electron pairs can be classified into bonding pairs and lone pairs. Bonding pairs are shared between atoms, while lone pairs are not involved in bonding. The repulsion between lone pairs is generally stronger than that between bonding pairs, resulting in different molecular shapes. For instance, if a molecule has four bonding pairs and no lone pairs, it adopts a tetrahedral shape. However, if there are three bonding pairs and one lone pair, the shape becomes trigonal pyramidal.
VSEPR theory is instrumental in predicting molecular geometry and understanding how different factors influence molecular shape. It provides a systematic approach to analyzing the arrangement of electron pairs and their impact on the overall geometry of a molecule.
How Do Bond Angles Influence Geometry?
Bond angles are a critical aspect of molecular geometry, as they determine the spatial arrangement of atoms in a molecule. These angles are influenced by the repulsion between electron pairs, with each type of geometry having characteristic bond angles. Understanding bond angles is essential for predicting molecular shape and properties.
In a linear geometry, the bond angle is 180°, resulting in a straight-line arrangement of atoms. Trigonal planar geometry features bond angles of 120°, forming a flat, triangular shape. Tetrahedral geometry, one of the most common shapes, has bond angles of 109.5°, creating a three-dimensional structure.
The presence of lone pairs can also affect bond angles, as they exert greater repulsive forces than bonding pairs. For example, in water (H₂O), the bond angle is 104.5° due to the repulsion from two lone pairs on the oxygen atom. Understanding bond angles is crucial for predicting how molecules interact and react, as they influence molecular polarity and reactivity.
Tetrahedral Geometry: A Common Shape
Tetrahedral geometry is one of the most prevalent molecular shapes, characterized by four bonding pairs around a central atom. This arrangement results in bond angles of 109.5°, forming a symmetrical, three-dimensional shape. Tetrahedral geometry is found in many organic and inorganic compounds, making it a fundamental concept in chemistry.
Common examples of tetrahedral geometry include methane (CH₄), where four hydrogen atoms are symmetrically arranged around a central carbon atom. This shape allows for equal distribution of electron density, leading to non-polar molecules. Tetrahedral geometry is also observed in ammonium ions (NH₄⁺) and various silicate minerals.
Understanding tetrahedral geometry is essential for predicting molecular behavior and interactions, as it provides insights into how molecules will align and react with other substances. This geometry is foundational for exploring more complex molecular structures and their properties.
Linear Geometry: Simplicity in Structure
Linear geometry is characterized by a straight-line arrangement of atoms, resulting in a bond angle of 180°. This simple shape is commonly found in diatomic molecules and some polyatomic molecules, making it an essential concept in molecular geometry.
Examples of linear geometry include carbon dioxide (CO₂) and hydrogen cyanide (HCN), where the central atom is bonded to two other atoms in a straight line. This arrangement is often associated with non-polar molecules, as the symmetrical distribution of electron density leads to a balanced charge distribution.
Linear geometry is fundamental for understanding molecular interactions and reactivity, as the straight-line arrangement influences how molecules align and bond with others. It provides a basis for exploring more complex molecular shapes and their properties.
Trigonal Planar Geometry: A Flat Arrangement
Trigonal planar geometry is characterized by three bonding pairs arranged in a flat, triangular shape around a central atom. This geometry features bond angles of 120°, resulting in a symmetrical, two-dimensional structure. Trigonal planar geometry is commonly found in molecules with double bonds, as the presence of a pi bond restricts rotation and maintains a planar shape.
Examples of trigonal planar geometry include boron trifluoride (BF₃) and ethylene (C₂H₄), where the central atom is bonded to three other atoms in a flat arrangement. This geometry often results in non-polar molecules, as the symmetrical distribution of electron density leads to a balanced charge distribution.
Understanding trigonal planar geometry is essential for predicting molecular behavior and interactions, as it provides insights into how molecules will align and react with other substances. This geometry is foundational for exploring more complex molecular structures and their properties.
Bent Geometry: The Influence of Lone Pairs
Bent geometry is characterized by a V-shaped arrangement of atoms, resulting from the presence of lone pairs on a central atom. This geometry features bond angles that are less than 120° or 109.5°, as the repulsion from lone pairs affects the overall shape. Bent geometry is commonly found in molecules with two bonding pairs and one or two lone pairs.
Examples of bent geometry include water (H₂O) and sulfur dioxide (SO₂), where the presence of lone pairs on the central atom results in a bent shape. This geometry often leads to polar molecules, as the asymmetrical distribution of electron density creates an imbalance in charge distribution.
Understanding bent geometry is crucial for predicting molecular behavior and interactions, as it provides insights into how molecules will align and react with other substances. This geometry is foundational for exploring more complex molecular structures and their properties.
Octahedral Geometry: A Six-Pointed Configuration
Octahedral geometry is characterized by six bonding pairs arranged symmetrically around a central atom, resulting in a three-dimensional shape with bond angles of 90°. This geometry is commonly found in transition metal complexes, where the central atom is surrounded by six ligands.
Examples of octahedral geometry include sulfur hexafluoride (SF₆) and the hexamminecobalt(III) ion ([Co(NH₃)₆]³⁺), where the central atom is bonded to six other atoms or groups in a symmetrical arrangement. This geometry often results in non-polar molecules, as the symmetrical distribution of electron density leads to a balanced charge distribution.
Understanding octahedral geometry is essential for predicting molecular behavior and interactions, as it provides insights into how molecules will align and react with other substances. This geometry is foundational for exploring more complex molecular structures and their properties.
Trigonal Bipyramidal Geometry: An Expanded View
Trigonal bipyramidal geometry is characterized by five bonding pairs arranged around a central atom, resulting in a three-dimensional shape with bond angles of 120° and 90°. This geometry is commonly found in molecules with expanded valence shells, where the central atom can accommodate more than eight electrons.
Examples of trigonal bipyramidal geometry include phosphorus pentachloride (PCl₅) and the phosphate ion (PO₄³⁻), where the central atom is bonded to five other atoms or groups in a symmetrical arrangement. This geometry often results in polar molecules, as the asymmetrical distribution of electron density creates an imbalance in charge distribution.
Understanding trigonal bipyramidal geometry is crucial for predicting molecular behavior and interactions, as it provides insights into how molecules will align and react with other substances. This geometry is foundational for exploring more complex molecular structures and their properties.
Molecular Geometry in Complex Molecules
Molecular geometry becomes increasingly complex in larger molecules, where multiple atoms and electron pairs contribute to the overall shape. In such cases, the geometry of individual atoms or groups within the molecule must be considered, as they can influence the molecule's behavior and interactions.
For example, in proteins, the geometry of amino acids and their side chains plays a crucial role in determining the protein's three-dimensional structure and function. Similarly, in nucleic acids, the geometry of nucleotide bases and sugar-phosphate backbones influences the formation of double helices and other secondary structures.
Understanding molecular geometry in complex molecules is essential for predicting biological activity and interactions, as it provides insights into how molecules will align and react with other substances. This knowledge is foundational for exploring more advanced topics in chemistry and biochemistry.
How Does Geometry Affect Molecular Polarity?
Molecular polarity is determined by the distribution of electron density within a molecule, which is influenced by its geometry. A molecule is polar if it has an uneven distribution of electron density, resulting in a dipole moment. This occurs when there is an asymmetrical arrangement of atoms and electron pairs, creating regions of partial positive and negative charge.
For example, in water (H₂O), the bent geometry and presence of lone pairs lead to an asymmetrical distribution of electron density, resulting in a polar molecule. In contrast, carbon dioxide (CO₂) has a linear geometry, leading to a symmetrical distribution of electron density and a non-polar molecule.
Understanding how geometry affects molecular polarity is crucial for predicting molecular behavior and interactions, as polarity influences solubility, reactivity, and intermolecular forces. This knowledge is foundational for exploring more advanced topics in chemistry and biochemistry.
Applications of Molecular Geometry in Real-World Scenarios
Molecular geometry has numerous applications in real-world scenarios, as it plays a crucial role in determining the properties and behavior of substances. In pharmacology, understanding the geometry of drug molecules is essential for predicting their interactions with biological targets, such as enzymes and receptors. This knowledge informs the design and development of new drugs with improved efficacy and safety.
In materials science, molecular geometry influences the properties of materials, such as their strength, flexibility, and conductivity. By understanding the geometry of polymer chains, scientists can design materials with specific properties for use in various applications, such as packaging, electronics, and textiles.
In environmental chemistry, molecular geometry is crucial for predicting the behavior of pollutants in the environment. Understanding the geometry of pollutants helps scientists assess their reactivity, persistence, and potential impact on ecosystems and human health.
Overall, molecular geometry is a fundamental concept with wide-ranging applications in science and industry, providing valuable insights into the behavior and interactions of molecules.
Challenges in Determining Molecular Geometry
Determining molecular geometry can be challenging, as it requires a detailed understanding of the arrangement of atoms and electron pairs within a molecule. Various factors can complicate this process, including the presence of multiple bonds, resonance structures, and steric hindrance.
Multiple bonds, such as double and triple bonds, can affect molecular geometry by altering bond angles and introducing rigidity into the molecule. Resonance structures, which involve the delocalization of electrons, can also complicate the determination of geometry, as they may result in intermediate shapes that are difficult to predict.
Steric hindrance, which occurs when atoms or groups within a molecule are too close together, can influence molecular geometry by altering bond angles and introducing strain into the molecule. This can complicate the prediction of geometry, as it requires consideration of both electronic and steric factors.
Despite these challenges, advances in computational chemistry and spectroscopy have provided powerful tools for determining molecular geometry, allowing scientists to explore complex molecules and their properties in greater detail.
FAQs
- What is the difference between molecular and electron geometry?
Molecular geometry refers to the arrangement of atoms in a molecule, while electron geometry considers the arrangement of all electron pairs, including lone pairs. - How does the VSEPR theory help predict molecular geometry?
The VSEPR theory predicts molecular geometry by minimizing the repulsion between electron pairs in the valence shell of the central atom, allowing for the most stable arrangement. - Why are bond angles important in molecular geometry?
Bond angles determine the spatial arrangement of atoms in a molecule, influencing its overall shape and properties, such as polarity and reactivity. - How does molecular geometry affect molecular polarity?
Molecular geometry affects polarity by influencing the distribution of electron density within a molecule, creating regions of partial positive and negative charge. - What are some real-world applications of molecular geometry?
Molecular geometry is used in pharmacology for drug design, in materials science for developing new materials, and in environmental chemistry for assessing pollutant behavior. - What challenges are associated with determining molecular geometry?
Challenges include the presence of multiple bonds, resonance structures, and steric hindrance, which can complicate the prediction of molecular geometry.
Conclusion
Molecular and electron geometry are fundamental concepts in chemistry, providing essential insights into the shape and structure of molecules. By understanding these geometries, scientists can predict how molecules will interact, react, and behave in different environments. This knowledge is crucial for various fields, including pharmacology, materials science, and environmental chemistry, where molecular geometry plays a vital role in determining the properties and functions of substances. Despite the challenges associated with determining molecular geometry, advances in computational chemistry and spectroscopy continue to enhance our understanding of this critical area, offering new opportunities for exploration and discovery in the world of chemistry.
Article Recommendations

